Do you know what’s in your water? You may be pleasantly surprised with what you’ve been drinking!
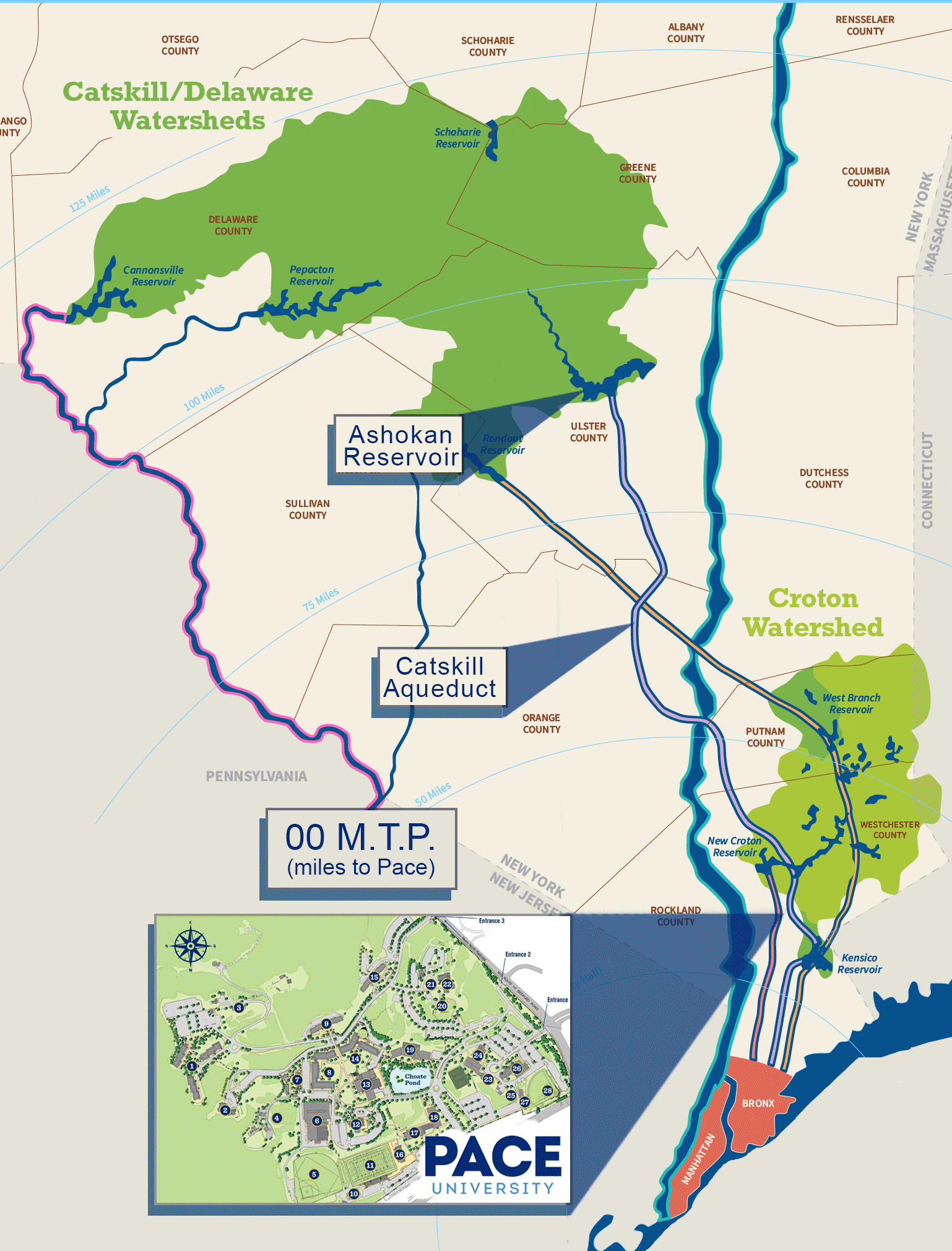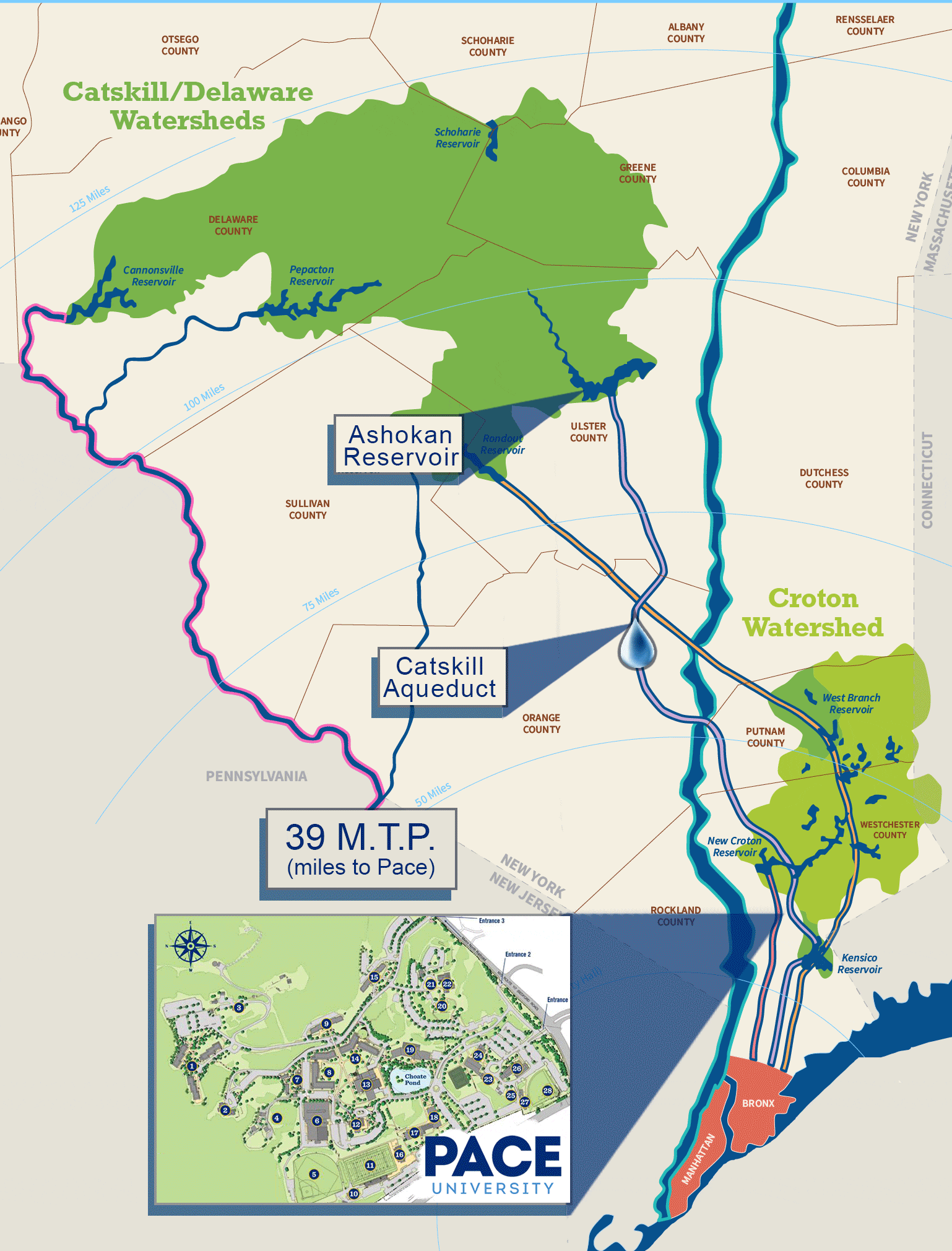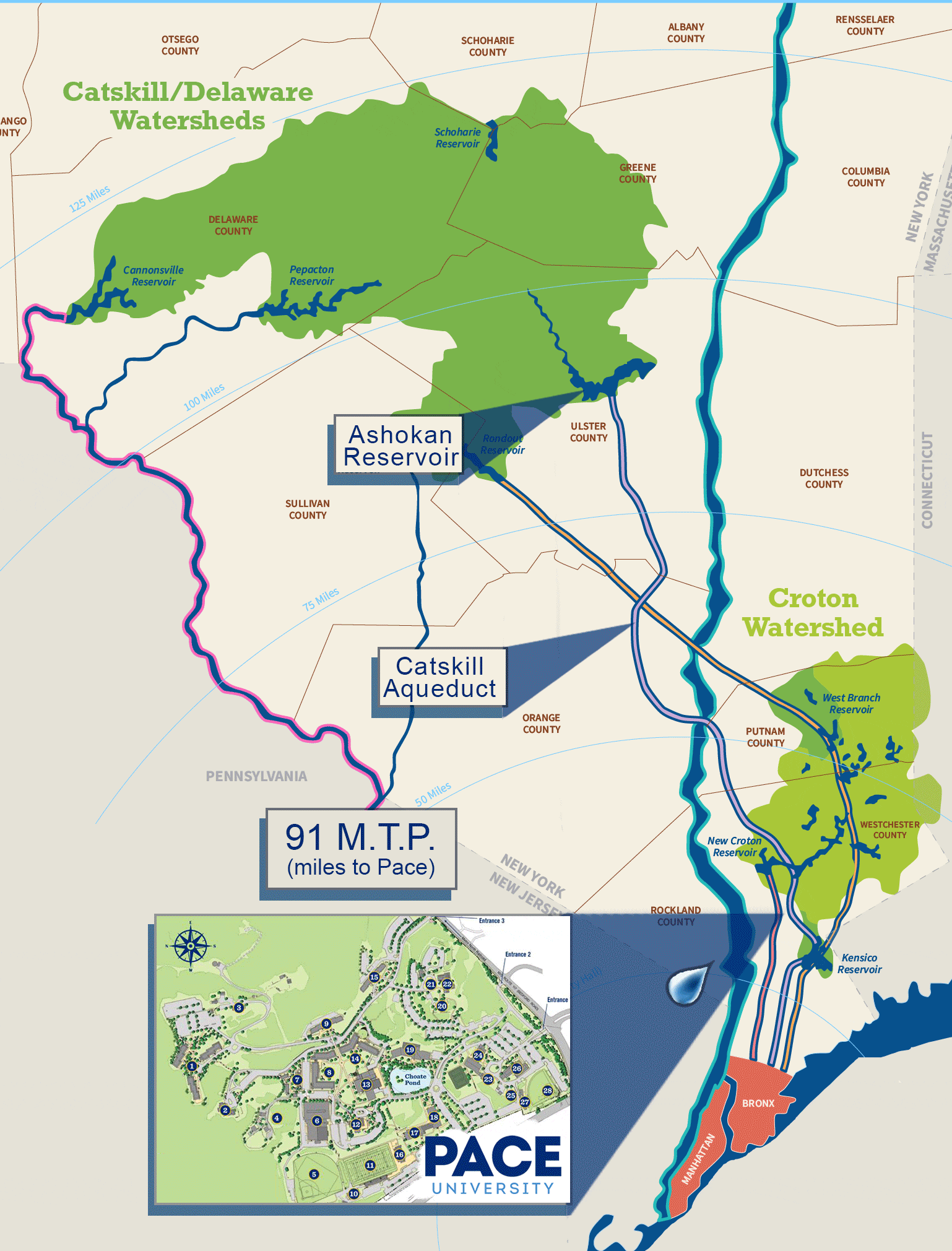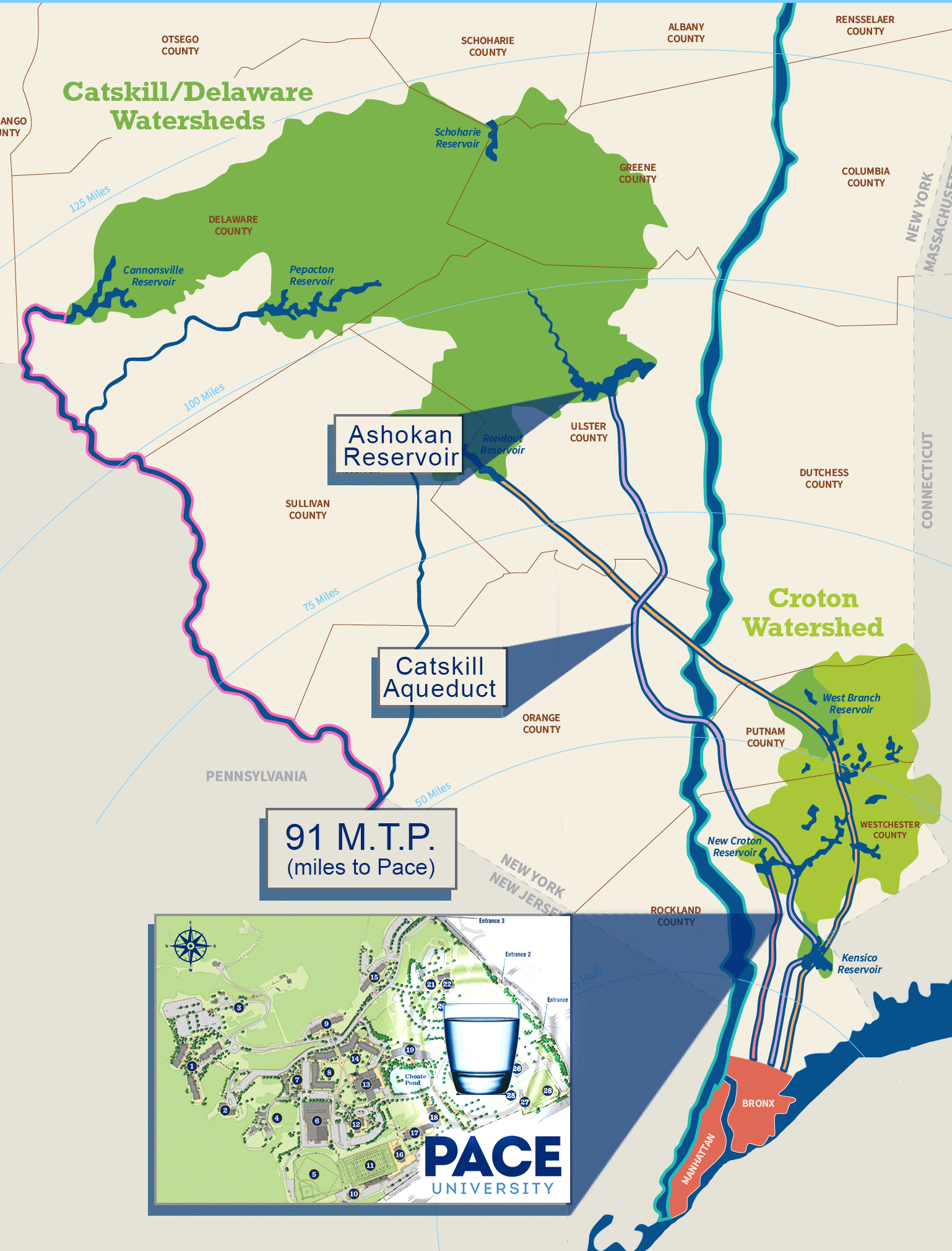
Our water is purchased from the Town of Mount Pleasant which purchases its water from the Town of New Castle Water District, whose primary source is the Catskill Aqueduct System and secondary source is the Croton Aqueduct System. The water originates from the Catskills located approximately 100 miles away from Pace University, then flows down the west side of the Hudson River and finally, underneath the river through a system of large pipelines to Mount Pleasant. Pace makes use of New York City’s water supply which serves approximately 7 million people. Pleasantville, as well as many other towns along the aqueduct to NYC, have the right to tap into that water supply.
This page meets your ‘right-to-know’ drinking water quality by describing the journey our water takes and information about its quality and current regulations. Answers to commonly asked questions about our water may be found in the “FAQ” tab below. To find Pace University Pleasantville’s yearly water quality reports dating back to 2017, click on the “Annual Reports” tab. Explanations on the contaminants tested are found under the “Contaminant Info” tab. For legal regulations and resources used to monitor water quality, click on the “EPA Manuals” tab.
To comply with State and Federal drinking water regulations, Pace University must issue an annual report on May 31st that provides water quality data for the previous calendar year (January – December) describing the quality of the campus’ drinking water. The purpose of the reports is to enable consumers to make informed decisions about their health as it relates to the consumption of water, and to raise our understanding of drinking water and awareness of the need to protect our drinking water sources.
In general, the sources of drinking water (both tap water and bottled water) include rivers, lakes, streams, ponds, reservoirs, springs, and wells. As water travels over the surface of the land or through the ground, it dissolves naturally occurring minerals and, in some cases, radioactive material, and can pick up substances resulting from the presence of animals or human activities. Contaminants that may be present in source water include microbial contaminants; inorganic contaminants; pesticides and herbicides; organic chemical contaminants; and radioactive contaminants. In order to ensure that tap water is safe to drink, the State and the EPA prescribe regulations that limit the number of certain contaminants in water provided by public water systems.
Who is responsible for maintenance of the water supply?
The water & wastewater specialists of Allied Pollution Control Inc. are Pace University’s water system operators. Pace’s water source is purchased from the Town of Mount Pleasant which purchases its water from the Town of New Castle Water District, whose primary source is the Catskill Aqueduct System and its secondary is the Croton Aqueduct System.
How and where is water sampled? What frequency?
The water is sampled at the New Castle Water District. The water is sampled by having ten samples collected from our water system. On a frequency basis, the State allows us to test for some contaminants less than once per year because the concentrations of these contaminants do not change frequently. Some of our data, though representative, are more than one year old. Field turbidity readings are recorded 5 days a week. The samples for these in-house testing are taken from varying locations on-site. Testing for total coliform is done 3 times a month by the State. Throughout the month, the three tasting dates fall between the 1 – 7th, 8 – 14th, and 15 – 21st of the month. Disinfection byproducts are tested 4 times a month, with samples taken at two different sites. Lead and copper may be tested every 6 months. However, if no contamination is found, the testing frequency would change to annual testing or testing every 3 years if there continues to be no contamination. Since Pace is a recipient of water that is already treated, we are not required to monitor additional contaminants.
What is water tested for?
Pace University is required to monitor for total coliform, lead, copper, total trihalomethanes, haloacetic acids, and turbidity. Additional contaminants are monitored in the New Castle Water District, including microbiological contaminants, inorganic compounds, volatile organic compounds, radiological compounds, and synthetic organic compounds.
What are the regulations Pace has to follow?
Regulations under the New York State Sanitary Code and the Federal Safe Drinking Water Act require Pace University to routinely test your drinking water for numerous contaminants.
What is Pace’s Water Emergency Management & Response plan?
Pace University can send notifications through Pace Alert, the university’s emergency notification system (via e-mail, text, and Pace Safe App). Students, staff, and faculty are automatically enrolled.
How can I stay informed about water quality emergencies?
Annual Water Quality Reports are published by Facilities and Capital Projects every May. In the future, this page will display updated water quality data so you can make informed decisions.
What should I do in the event of a water emergency (i.e. where can I report concerns?)
In the event of a water emergency, if you have any questions concerning your drinking water, please contact the Pace University Facilities Management Office at (914) 923-2840. We want you to be informed about your drinking water. To obtain additional information on the drinking water provided to Pace University, you may contact the Mount Pleasant Water District directly, at 119 Lozza Drive, Valhalla, New York 10595-1268, or by telephone at (914) 742-2313.
What are regular precautions I should take?
If the faucet or showerhead has not been used recently, defined as a week or more, the CDC recommends flushing them before use. It is recommended that you run the cold water for two minutes; then switch to hot water until the water starts to feel hot. Regularly clean any device that uses water. There are steps that you as a Pace Community member can do to protect our water source. Don’t dispose of hazardous waste (i.e. mothballs, cleaners, medication) down your sink, on the ground, or in storm sewers. Help clean up local water sources.
What are my rights?
Since 2010 the UN has declared that clean water is a human right. Furthermore, one needs to know what’s in their water to make informed decisions, a human right recognized by the UN in 2015. You can read more about this on the UN’s website. Current federal regulations only require reports to be posted yearly – having water quality that could be a year old can not be used to make informed decisions. Blue CoLab seeks to give regular reports so community members can actively make informed decisions on their water.
Where does wastewater go?
Wastewater from most of the Pocantico River watershed goes to the Yonkers plant, which is the largest contributor of liquid waste/sewage to the Lower Hudson and is also a combined sewer system.

Pace University Pleasantville is required to issue a yearly water quality report because its drinking water system is considered a “community water system” within the meaning set forth in the State Sanitary Code and the Federal Safe Drinking Water Act. All findings from each of the below analyses are contained in the Report and presented in tables divided in different sections pertaining to the type of contaminant, the date of each sample, and whether the testing of a given contaminant revealed a violation of New York State drinking water regulations.
For an explanation on the contaminants listed in the reports, see the “Contaminant Information” tab.
The drinking water analyses conducted for the Pace University Pleasantville campus during the January 1, 2021 to December 31, 2021 period, and presented in that year’s Annual Water Quality Report, found that all results were in accordance with the water quality regulations set forth in the New York State Department of Health Sanitary Code.
The drinking water analyses conducted for the Pace University Pleasantville campus during the January 1, 2020 to December 31, 2020 period, and presented in that year’s Annual Water Quality Report, found that all results were in accordance with the water quality regulations set forth in the New York State Department of Health Sanitary Code.
Pace Water Quality Report for 2019 (PDF )
The drinking water analyses conducted for the Pace University Pleasantville campus during the January 1, 2019 to December 31, 2019 period, and presented in that year’s Annual Water Quality Report, found that all results were in accordance with the water quality regulations set forth in the New York State Department of Health Sanitary Code.
Pace Water Quality Report for 2018 (PDF )
The drinking water analyses conducted for the Pace University Pleasantville campus during the January 1, 2018 to December 31, 2018 period, and presented in that year’s Annual Water Quality Report, found that all results were in accordance with the water quality regulations set forth in the New York State Department of Health Sanitary Code.
Pace Water Quality Report for 2017 (PDF )
The drinking water analyses conducted for the Pace University Pleasantville campus during the January 1, 2017 to December 31, 2017 period, and presented in that year’s Annual Water Quality Report, found that all results were in accordance with the water quality regulations set forth in the New York State Department of Health Sanitary Code.
Below are common contaminants tested for in the Pace Water Quality Reports. Contaminants include total coliform, lead, copper, total trihalomethanes, haloacetic acids, and turbidity. The description of the contaminant, how it is tested, and its effect on health are explained for each. Words with numerical citations are defined below the list of contaminants.
Total Coliform
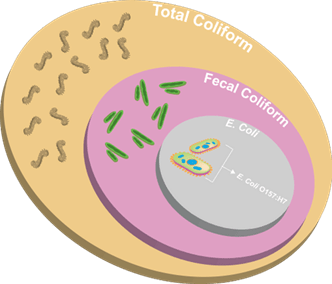
Description: Total coliforms are a group of bacteria that includes non-fecal coliform (found in soil and surface water) and fecal coliform. Fecal coliform is a subset of coliform that is heat tolerant and found in animal waste and human sewage. This group consists of Escherichia coli (E. coli), which is a bacteria that is commonly found in the intestines of animals and humans.
How it’s Tested: A method of determining bacteria contamination in water is to count the number of bacteria colonies that grow on a prepared medium. The number of total coliform bacteria is widely used as an indicator for drinkable water in the United States. E. coli present in water is a strong indicator of sewage or animal waste contamination. Total coliforms were originally believed to indicate the presence of fecal contamination, but they have been found to be widely distributed in nature, not always associating with the gastrointestinal tract of warm-blooded animals. However, high numbers of even harmless bacteria can indicate high numbers of harmful bacteria, viruses, or protozoa¹, as well.
Effect on Health: Sewage and animal waste can contain organisms that cause disease, which may result in severe illness if consumed. While minor gastrointestinal discomfort is one of the most common symptoms, pathogens that cause only minor sickness in some people may cause serious conditions or death in others, especially in the very young, old, or those with weakened immune systems.
Lead

Description: The likely source of lead contamination in water is from corrosion of household plumbing systems and erosion of natural deposits. It is possible that levels of lead at different homes in the community may be higher than others as a result of materials used in the home’s plumbing. Pace University’s water system is responsible for providing high-quality drinking water but cannot control the variety of materials used in plumbing components. To minimize the potential for lead exposure, you can flush your tap for 30 seconds to 2 minutes before using water for drinking or cooking.
How it’s Tested: The Safe Drinking Water Act requires the U.S. Environmental Protection Agency (EPA) to set up goals for the level of contaminants in drinking water below which there is no known or expected risk to health with an adequate margin of safety. These goals are solely based on possible health risks and are called maximum contaminant level goals (MCLGs)². EPA has set the MCLG for lead in drinking water at zero because even at low exposure levels, lead is a toxic metal that can be harmful to human health.
Effect on Health: Lead is persistent and can accumulate in the body over time. Elevated levels of lead can cause serious health problems, especially for pregnant women, infants, and young children. In children, low levels of exposure have been linked to damage to the central and peripheral nervous system, learning disabilities, slowed growth, impaired hearing, and impaired formation and function of blood cells such as anemia.
Copper

Description: Like lead, copper primarily enters drinking water through the corrosion of plumbing materials and erosion of natural deposits. Additionally, copper enters the environment from industrial and domestic waste, mining, and mineral leaching.
How it’s Tested: Similar to lead, the U.S. Environmental Protection Agency (EPA) must determine a maximum contaminant level goal (MCLG)² for copper in water. If the concentration of copper exceeds an action level of 1.3 parts per million (ppm) in more than 10% of the taps sampled, a number of additional actions must be undertaken to control corrosion. Action level (AL)³ is the concentration of a contaminant that, if exceeded, triggers treatment or other requirements that a water system must follow.
Effect on Health: Exposure to copper may cause stomach and intestinal distress, liver and kidney damage, and in high doses, anemia and brain damage.
Haloacetic Acids
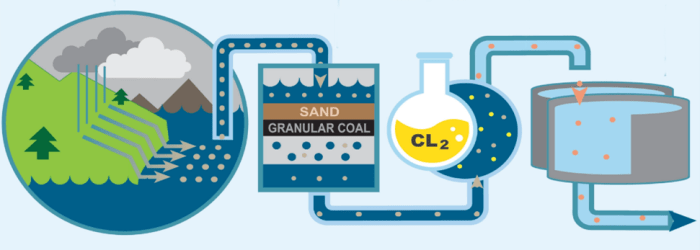
Description: Similar to trihalomethanes, haloacetic acids (HAA5) are disinfection byproducts³ and include the individual chemicals chloroacetic acid, dichloroacetic acid, trichloroacetic acid, bromoacetic acid, and dibromoacetic acid. This group of chemicals is formed in drinking water during disinfection when chlorine reacts with naturally occurring organic material in water sources such as rivers and lakes.
How it’s Tested: Chlorination of drinking water is needed to kill harmful organisms such as bacteria and viruses that could cause serious illnesses. However, factors such as the amount of organic matter in water sources, temperature, and the amount of chlorine added affect the amount of HAA5 formed. Specifically, the source of potential HAA5 in Pace’s drinking water would come from the chlorination of water provided by the Mount Pleasant Water District. The U.S. Environmental Protection Agency (EPA) sets a maximum contaminant level goal² of 0.06 parts per million (equivalent to 0.06 milligrams per liter) for HAA5.
Effect on Health: In regards to health, some studies suggest that drinking chlorinated water containing HAA5 for long periods of time, such as 20 to 30 years, can lead to an increased risk of cancer, low birth weight, miscarriage, birth defects, and other health effects. However, the evidence in these studies is not strong enough to conclude that HAA5s were a major factor contributing to the observed increased risks. Compared to the risks for illness from drinking inadequately disinfected water, the risks for adverse health effects from HAA5s in drinking water are small.
Total Trihalomethanes

Description: Trihalomethanes (TTHMs) are disinfection byproducts³ and include the individual chemicals chloroform, bromoform, bromodichloromethane, and chlorodibromomethane. Disinfection byproducts are formed in drinking water during disinfection when chlorine reacts with naturally occurring organic material in water sources such as rivers and lakes.
How it’s Tested: The U.S. Environmental Protection Agency (EPA) sets a maximum contaminant level goal² of 0.08 parts per million (equivalent to 0.08 milligrams per liter) for TTHMs. Chlorination of drinking water is needed to kill harmful organisms such as bacteria and viruses that could cause serious illnesses. However, factors such as the amount of organic matter in water sources, temperature, and the amount of chlorine added affect the amount of TTHMs formed. Specifically, the source of potential TTHM in Pace’s drinking water would come from the chlorination of water provided by the Mount Pleasant Water District.
Effect on Health: In regards to health, some studies suggest that drinking chlorinated water containing TTHMs for long periods of time, such as 20 to 30 years, can lead to an increased risk of cancer, low birth weight, miscarriage, birth defects, and other health effects. However, the evidence in these studies is not strong enough to conclude that TTHMs were a major factor contributing to the observed increased risks. Compared to the risks for illness from drinking inadequately disinfected water, the risks for adverse health effects from TTHMs in drinking water are small.
Turbidity
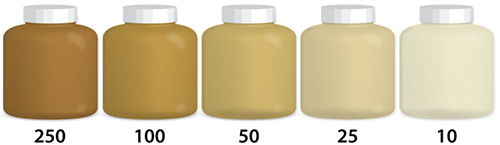
Description: Turbidity is the measure of the relative clarity of water. The greater the suspended particles, the higher the level of turbidity and the cloudier the water. Materials that cause water to be turbid include clay, silt, very tiny inorganic and organic matter, algae, dissolved colored organic compounds, and plankton and other microscopic organisms. Some pollutants, such as metals, insoluble toxins, and bacteria, can attach themselves to suspended particles. The particles in cloudy water provide “shelter” for microbes by reducing their exposure to disinfectants. A likely source of contamination that leads to higher turbidity levels is soil runoff.
How it’s Tested: Turbidity is measured in Nephelometric Turbidity Units (NTU) by shining a light through a sample of water. The higher the NTU, the more cloudy/opaque the water is. New York State’s maximum regulatory contamination level⁵ limit is 5 NTU.
Effect on Health: Turbidity readings are a good indicator of water quality and potential pollution. The same conditions that cause high turbidity can promote the regrowth of pathogens, such as bacteria and viruses, leading to waterborne disease outbreaks, which have caused intestinal sickness throughout the United States and the world.
Definitions
- [1] Protozoa – A group of single-celled eukaryotes that are either free-living or parasitic. They feed on organic matter such as other microorganisms or organic tissues and debris.
- [2] Maximum Contaminant Level Goal (MCLG) – The level of a contaminant in drinking water below which there is no known or
expected risk to health. The Safe Drinking Water Act requires the U.S. Environmental Protection Agency (EPA) to set up these goals of the maximum concentration of a contaminant with an adequate margin of safety. These goals are solely based on possible health risks. - [3] Action level (AL) – The concentration of a contaminant which, if exceeded, triggers treatment or other requirements that a water system must follow.
- [4] Disinfection Byproduct – Chemicals formed during disinfection of drinking water containing suspended or dissolved organic material. The process of disinfection is when water systems add chlorine to drinking water to eliminate harmful organisms. During this process, chlorine also reacts with naturally occurring organic material (e.g., decomposing vegetation such as tree leaves, algae, or other aquatic plants) that may be present in drinking water. Organic compounds may come from water sources such as rivers and lakes. The toxic compounds created during the water purification process may negatively affect the health of those with compromised immune systems and the elderly.
- [5] Maximum Contaminant Level (MCL) – The highest level of a contaminant that is allowed in drinking water. MCLs are set as close to the MCLGs as feasible.
In the EPA manual, you will find the regulations and resources used to monitor water quality published by the United States Environmental Protection Agency (EPA). Federal requirements for drinking water, water quality standards in your area, and additional resources for research are all included on this helpful website.
The Laboratory Certification Manual for Drinking Water describes implementation procedures, laboratory procedures, and the technical criteria for laboratories that analyze samples of drinking water for compliance. Certified laboratories must use approved methods to analyze drinking water samples for compliance from public water systems (PWS). This to ensure that PWSs are provided with reliable information regarding the quality of their water, which then enables the average consumer to make informed decisions about their health.
Laboratories must:
- Be certified by EPA or the state to analyze drinking water samples for compliance monitoring
- Successfully analyze proficiency testing (PT) samples at least annually for each method and analyze for which they desire certification
- Use approved methods
- Pass periodic on-site audits
Per- and polyfluoroalkyl substances (PFAS) are a large class of synthetic chemicals that present numerous analytical challenges. The EPA has various methods for analyzing PFAS in environmental media that are in differing stages of development and validation. EPA scientists are not only developing analytical methods for drinking water, but also ground water, surface water, wastewater, and more. These may eventually all become standard methods for research, meaning that they have been through a laboratory validation process following a particular rulemaking or guidance effort and are available to support regulatory or guidance activities.
